Maximum purity. Perfect flow control.
Optimum process control.Contamination-free flow measurement in biotech cleanrooms
Maximum safety and purity flow visualization for biotechnology and the pharmaceutical industry
Controlled cleanroom conditions are essential in biotechnology and pharmaceutical production in order to guarantee sterile processes and ensure the quality of sensitive products such as medicines, vaccines or cell cultures.
Even the smallest air turbulence or uncontrolled flow can cause cross-contamination and render entire production batches unusable. Precise air flow control is therefore of the utmost importance.
With the FlowBOS CR camera, LaVision offers an innovative solution for particle-free visualization of air flows in laboratories and cleanrooms for the biotech industry – without contamination, without long interruptions in operation and without residues.
Flow control in cleanrooms: Why precision measurements are essential in the biotech industry
Choosing the right airflow concept is crucial for sterility and efficiency in pharmaceutical production rooms,
cleanroom laboratories and cell factories. Different applications require specific flow concepts to ensure product quality and safety.
Laminar flow zones
Homogeneous air flow to avoid particle inclusions
Low-turbulence displacement flow (TAV)
Ensuring sterile conditions through directed air flow
Hybrid solutions
Combination of mixed flow and targeted airflow for cleanroom zones with variable requirements
LaVision the perfect partner for cleanroom flow measurements in the biotech industry
FlowBOS CR camera - Particle-free air flow analysis
for sterile production environments
FlowBOS camera for biotech cleanrooms. LaVision’s FlowBOS CR biotech cameras enable high-precision flow visualization without using aerosols or smoke particles. Instead, the system uses a non-hazardous and optically active tracer gas that remains sterile and does not cause cross-contamination. This advanced BOS imaging technology allows airflows to be accurately mapped and analyzed in real time to identify contaminants and air turbulence before they become a problem. Flow visualization for biotech and pharma cleanrooms – Maximum hygiene, zero particle risk.
- Video recordings of the undisturbed air flow in real time
- Air and inert gas are physically identical in terms of flow with ideal subsequent behavior
- FlowBOS CR Biotech camera specially designed for use in pharmaceutical and biotechnological cleanrooms

Convincing features of FlowBOS measurement technology for cleanrooms in the biotech and pharmaceutical industries
More information about the product
LaVision FlowBOS CR camera
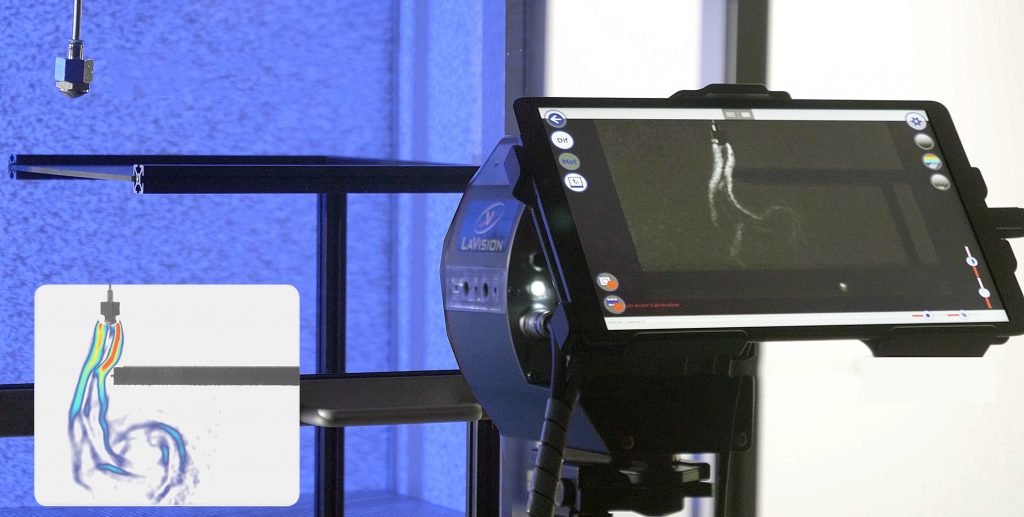
Comprehensive data processing and visualization options
Effective flow management in cleanrooms requires powerful analysis and visualization solutions to record, evaluate and optimize air flows in real time.
The analysis options of the FlowBOS system enable automated, precise recording and analysis of all relevant air flow parameters and provide the results visually in real time.
Precise visualization of air flows to reduce the risk of contamination
Integration of FlowBOS into existing certification and quality documentation processes
Questions & answers on the use of FlowBOS-CR
in BioTech cleanrooms
Why is optical flow visualization better than conventional smoke tests?
Conventional smoke tests can cause contamination. The FlowBOS CR-Camera enables residue-free analysis that can also be used safely in sterile production environments.
How can the FlowBOS CR-Camera increase safety in pharmaceutical production rooms?
Which flow concepts can be analyzed?
- Laminar flow for sterile areas
- Low-turbulence displacement flow (TAV) for contamination-free zones
- Hybrid flow for combined manufacturing processes
Is FlowBOS CR-Camera ideal for pharmaceutical & biotechnology production facilities?
The system meets GMP and FDA standards and has been specially developed for the requirements of sterile production areas.
How can the FlowBOS measuring method be integrated into existing quality control systems?
Thanks to intelligent software interfaces, the FlowBOS CR-Camera can be integrated into existing GMP quality management and monitoring systems.
